KidneyCell Commercial Plan
KidneyCell LTP a Leonhardt’s Launchpads innovation and startup launch accelerator Licensable Technology Platform (LTP) and startup.
Executive Summary
Product and services & the problem KidneyCell solves
KidneyCell has patented is developing a product pipeline of 3 products for total kidney regeneration and recovery…
1. KidneyCell TM = Non-invasive bioelectric regeneration promoting protein expression stimulation.
2. KidneyCell Plus Lite Biologics = Bioelectric stimulation + PRF and regenerative fluid (amniotic or exosomes).
3. Kidney Cell Plus Full Biologics = Bioelectric stimulation + proprietary fifteen component KC-15 kidney regeneration composition comprised of combination that may include adipose tissue derived stem cells and stromal fraction, hypoxia treated MSCs, PRF, exosomes, selected growth factors, nutrient hydrogel, Wharton’s Jelly, selected alkaloids, oxygenated nanoparticles, extracellular matrix.
Our patented and patent pending precise bioelectric signaling controlled kidney regeneration promoting protein expressions include; klotho, SDF1, PDGF, IGF1, VEGF, EGF, HGF, eNOS, HIF1a, tropoelastin, COL17A1, follistatin and sonic hedgehog. Many of these protein expressions especially klotho, SDF1, PDGF and IGF1 have been shown to be effective in kidney recovery studies.
No other product totally regenerates kidneys restoring them to full function for the balance of the patient’s life and relieving them of the need for a kidney transplant, drugs or dialysis.
Kidney transplants cost $110,000+ and people have to wait often 7 years on a waiting list. Kidney transplant patients have to take expensive anti-rejection drugs the rest of the their life with side effects. The cost of kidney procurement for kidney transplants is > $40,000 per kidney.
Dialysis costs > $50,000 a year or $500,000 over a 10 year course of treatment and the patients have a low quality of life.
Drugs for failing kidneys are ineffective, expensive and have many side effects…
Medicines for kidney patients
Anti-hypertensives (blood pressure tablets)
You might need anti-hypertensive tablets to lower your blood pressure.
Prolonged high blood pressure can damage your blood vessels, heart and kidneys. This might mean you need a transplant or dialysis sooner.
There are many different tablets available. Some of the most common ones prescribed for kidney patients are called Doxazosin, Atenolol, Ramipril and Irbesartan.
Reducing your blood pressure will not make you feel better in the short-term. However, in the long-term, the tablets will help you to stay healthy.
Diuretics (water tablets)
Healthy kidneys are very good at producing the right amount of urine to match the fluid you take into your body when you eat and drink. Damaged kidneys are not so good at producing urine. This may mean that fluid builds up in your body, causing swollen ankles, difficulty breathing and high blood pressure.
Diuretics are tablets which encourage your kidneys to produce more urine. This makes you go to the toilet more. The most common diuretic is Furosemide.
When taking diuretics it is important not to drink too much fluid because the medication will be less effective and you will need to take higher doses.
Erythropoietin (EPO)
Erythropoietin is often known as EPO. It is a hormone which is produced by healthy kidneys. EPO stimulates the bone marrow to make red blood cells. When your kidneys are not working properly you do not make enough EPO and you become anaemic. You may then feel tired, weak, cold and generally unwell.
In this situation you will be prescribed EPO injections. You can be taught how to do this yourself at home.
There are several brands of EPO available.
Hepatitis B vaccination
Anyone needing dialysis is at a slight risk of getting hepatitis B. This is a viral infection spread through infected blood or bodily fluids. Therefore you may be advised to have an hepatitis B vaccination.
Iron supplements
Iron is essential for making red blood cells. Without it you will become anaemic.
You may require iron supplement tablets. The most commonly prescribed tablet is ferrous sulphate.
People with CKD are often unable to absorb iron from their stomach and sometimes they will need iron injections.
Phosphate binders
These help to control your blood phosphate levels. The medication may be called Calcichew®, Adcal®, Fosrenol®, Renagel® or Phosex®. These need to be taken up to 15 minutes before, or with, food.
Reducing your phosphate levels can help to prevent itchiness and bone weakness. It may help reduce your risk of heart problems.
Sodium Bicarbonate
This is given to help prevent the build up of acid in your body.
If your kidneys function decreases there is less acid in your urine. This causes your bicarbonate level to drop. A low bicarbonate level can be bad for your heart and can hasten the deterioration of your kidney function.
Statins (cholesterol tablets)
You might need statins to lower your blood cholesterol level. High cholesterol can lead to heart disease.
There are many different brands of statins available including simvastatin, pravastatin and atorvastatin.
A healthy diet and exercise can also help to lower your cholesterol.
Vitamin D
This helps control calcium in your body and protects your bones.
The medication may be called alfacalcidol. You take this daily or weekly depending on the instructions.
Over the counter medicines and herbal remedies
Some medications that you can buy from a pharmacy or supermarket without a doctor’s prescription are not suitable for people with CKD. You should talk to your doctor or nurse before taking any of these medications or any herbal remedies.
KidneyCell has a goal via total kidney regeneration to save over $50,000 a year in dialysis costs and will avoid the $110,000 up front cost of a kidney transplant and ongoing costs for anti-rejection and other drugs.
Target Market
The kidney failure and renal disease market is > $93 billion annually worldwide – Click Here. Our product lineup is intended to treat all types and levels of kidney failure.
Will Dialysis Become a Thing of the Past? Discovery Kidneys can be Rejuvenated – Click Here
Competition
- Kidney transplants cost about $110,000 each in the USA with the kidney procurement portion costing > $40,000.
- Dialysis costs over $50,000 annually or $500,000 over a 10 year course of treatment.
- Drugs cost up to $40,000 a year and are ineffective with side effects.
- Stem cell therapies for kidney regeneration are a partial solution and cost > $15,000 per treatment – Click Here
Financial Overview
Below assumes partnering with large scale commercial partner with large sales force…
KidneyCell 5 Year Sales Forecast Post Full Market Launch
Year 1 = $15,000,000
Year 2 = $90,000,000
Year 3 = $180,000,000
Year 4 = $360,000,000
Year 5 = $720,000,000
KidneyCell Biologics (Lite and Full Biologics products combined) 5 Year Sales Forecast Post Full Market Launch
Year 1 = $90,000,000
Year 2 = $900,000,000
Year 3 = $1.8 billion
Year 4 = $2.8 billion
Year 5 = $3 billion
- KidneyCell TM cost = $250 Projected Retail Sell Price = $3000 to $15,0000 =
- KidneyCell Plus Lite Biologics cost = $1650 Projected Retail Sell Price = $6,000 to $25,000 ]
- KidneyCell Plus Full Biologics cost = $9250 Projected Retail Sell Price = $20,000 to $60,0000
- Cost of stimulators in volume = $250 for external, $800 for implantable
- Cost of micro infusion pumps in volume = $250 for external, $800 for implantable
- Cost of biologics lite composition in volume = $1400 per treatment
- Cost of full biologics composition in volume = $9000 per treatment
- Expected retail sales price non-invasive treatment = $3000 to $15,000 per patient
- Expected retail sales price bioelectric + lite biologics = $6,000 to $25,000 per patient
- Expected retail sales prices bioelectric + full biologics = $20,000 to $60,000 per patient
Regulatory Progress and Pathway
- KidneyCell benchtop stimulator has FDA 510K market clearance and CE Mark now for improving blood circulation and pain relief.
- KidneyCell portable stimulator is expected to have FDA 510K market clearance and CE mark later this year.
- KidneyCell Plus Lite Biologics can be sold now as PRF point of care centrifuges and regenerative fluid have market clearances in USA.
- KidneyCell Plus Full Biologics will require a BLA. The implantable stimulators have a master file with the FDA already. FDA and CE mark cleared pumps may be purchased from Prometra or Medtronic Synchromed now.
- Full proper FDA Biologics License Approval (BLA) for full therapy is expected in 2022.
Business Model
The KidneyCell business model is to seek a strategic alliance with a large company partner immediately after having first in human clinical trial data in hand and patents issued.
Our Team
KidneyCell core team, board and advisors have brought multiple products from concept to market leadership. Our team completed our first stem cell based organ regeneration study in 1988 published in The Physiologist in 1989 working with our current team member Dr. Race Kao. In 1999 we published in Circulation our first bioelectric regenerative protein expression study working with Dr. Shinichi Kanno. In 2001 our team lead the completion of the landmark first muscle stem cell repair of a human heart in The Netherlands. We are guided by top level nephrologists and cardiologists as well as stem cell based regeneration scientists and experienced bioengineers.
- Dr. Samer Banihani a Nephrologist at Sacred Heart Providence Hospital in Spokane, WA is our Chief Nephrology Advisor
- Dr. Leslie Miller a Cardiologist with over 241 peer reviewed publications and experience with over 90 clinical trials is our Chief Medical Officer
- Phil Patton our President has over 20 years industry experience.
- Howard Leonhardt our Executive Chairman & CEO has brought multiple products from concept to market leadership in the medtech and biotech arena.
Our team > Click Here
Our Scientific Advisory Board > Click Here
Evidence of early success and supporting data
- Dozens of published studies have documented the role of klotho in kidney regeneration. We have patent pending the only known bioelectric signaling sequence to substantially increase klotho expression by up to 465%.
- Dozens of published studies have documented the roles of SDF1 and PDGF in stem cell homing, proliferation and organ regeneration and healing.
- A great number of studies have documented the beneficial effect of stem cell therapy on organ regeneration including kidney recovery.
- A growing number of studies have documented a positive role in applying selected exosomes to organ regeneration and recovery including kidney recovery.
- A number of studies have document a positive role for IGF1 in organ regeneration and healing including kidney recovery.
- Bioelectric stimulation has been documented in numerous studies to have a positive role in organ regeneration and healing including kidney recovery.
- Our research partners in Brazil previously published on the positive effects of bioelectric stimulation on reducing DNA damage in chronic kidney failure patients – Click Here
Patents
KidneyCell via Leonhardt’s Launchpads and Leonhardt Ventures LLC has exclusive ownership rights pertaining to kidney regeneration to over 600 patent claims including pioneering patent claims for stem cell homing and a combination stimulator + pump + composition therapy. One of KidneyCell’s most important patent’s pending is for bioelectric controlled klotho expression.
- Stimulator, Pump and Composition Issued U.S. Patent > Click Here
- Combination of Bioelectrical Stimulator and Platelet-Rich Fibrin for Accelerated Healing and Regeneration > Click Here
- Leonhardt’s Launchpads Announces Filing of Patent for Bioelectric Stimulation Controlled Klotho Expression ->
- Powerful Anti-aging and Regeneration Promoting Protein – Click Here
- Bioelectric Stimulator > Click Here
- System and Method for Treating Inflammation> Click Here
Upcoming Milestones
- 3Q 2020 – Completion of KidneyCell pilot clinical study in Brazil.
- 3Q 2020 – Completion of development of portable stimulator.
- 3Q 2020 – Completion of development of KC-15 kidney regeneration composition and launch of large animal studies for Kidney Plus Full Biologics.
- 4Q 2020 – Launch of KidneyCell Plus Lite Biologics clinical pilot study = bioelectric + regenerative fluid.
- 1Q 2021 – Completion of multi-center pivotal clinical study.
- 2Q 2021 – Exit to strategic alliance partner for full commercialization pursuit.
Funding Needs
- Pilot clinical study Brazil = $40,000
- Portable stimulator development = $30,000
- KC-15 composition development = $100,000
- KidneyCell Plus Lite Biologics Pilot Study = $150,000
- KidneyCell Pivotal Study = $350,000
$670,000 for 29.1% of company at $2,300,000 post money fully diluted valuation.
KidneyCell LTP (Licensable Technology Platform with Pre-Incorporation Rights)
- Current Price Per Unit LTP Share = $1
- Fully Diluted Valuation = $2,300,000
- 1,773,000 shares issued as of July 14th, 2020 = current pre-money valuation of $1,773,000
- 583,000 conditional warrants/share options granted to date
- 644,000 shares still available in inventory under original authorized total shares
- Total Authorized Shares = 2,300,000
- Investment is 2:1 via accelerator = If you invest $30K you get $30K of Leonhardt’s Launchpads by Cal-X Stars Business Accelerator, Inc. shares at $0.36585 a share = 82,001 shares AND FREE OF ADDITIONAL CHARGE also 30,000 share units of KidneyCell LTP (Licensable Technology Platform with Pre-Incorporation Rights) – see full Private Placement Memorandum, Annual Report and Investor FAQs page for all risk factors and other details.
- Reserved only for accredited sophisticated investors with experience in early stage biotech or medtech investments.
- Business Model = Sell to a strategic buyer under an Asset Purchase/Sale Agreement as a kidney regeneration focused Licensable Technology Platform in a milestone deal after first in human clinical results and patents are issued. Attempt to get a 3% royalty on sales if possible. Only if pathway to sell under an Asset Purchase/Sale Agreement is exhausted over a number of years will the group consider converting the LTP into a stand alone separate C corporation. If that conversion is made all LTP unit share stakeholders have Pre-Incorporation Rights to have exactly the same percentage ownership in common stock in the spun out C corporation at the time of conversion.
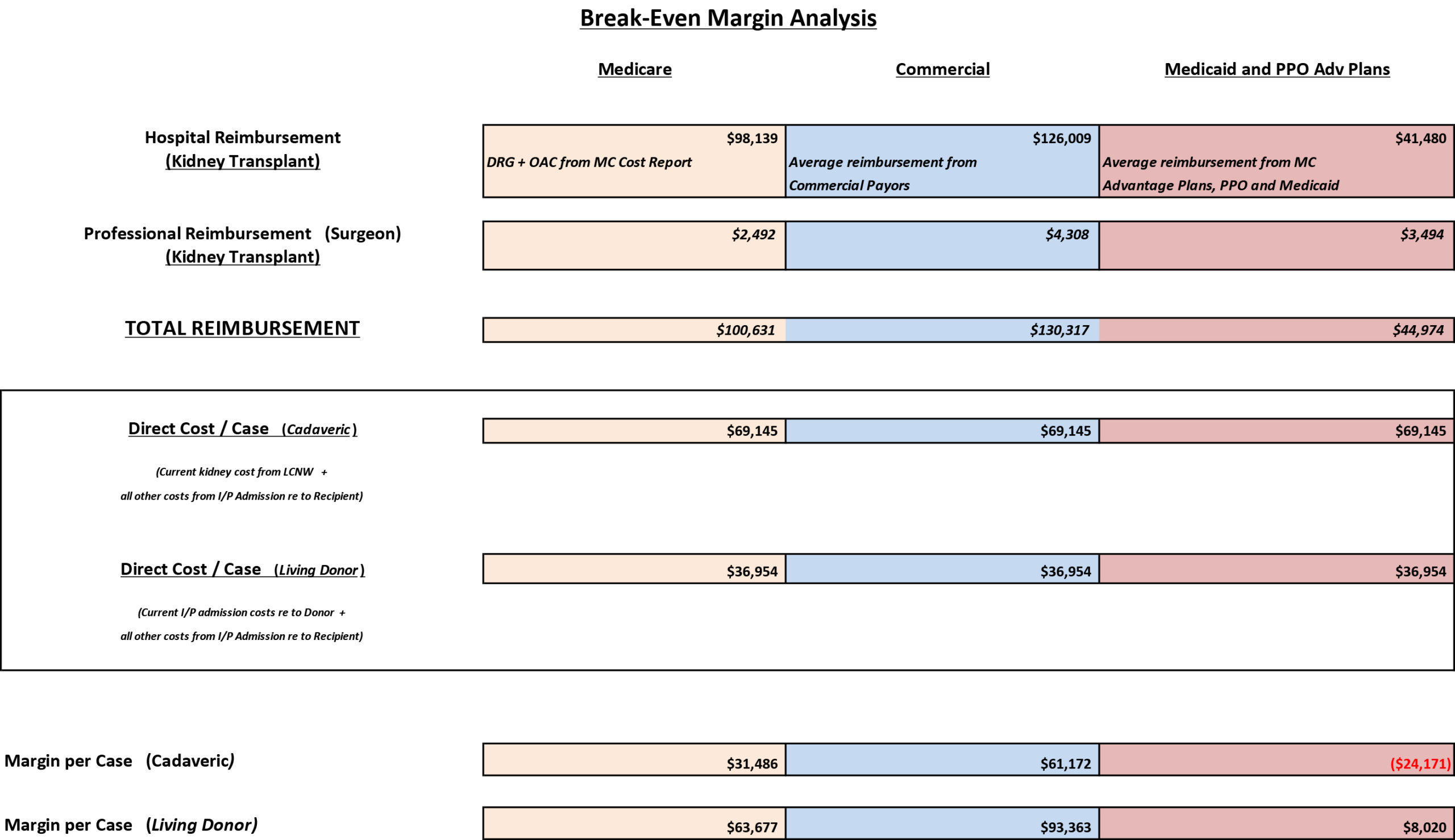
Reimbursement and Cost Analysis Kidney Transplants